Malaria remains one of the deadliest infectious diseases with more than 600,000 deaths worldwide each year, despite the decades of efforts to eradicate this mosquito-borne disease.
A patient becomes sick when the malaria parasites infect and replicate inside red blood cells, causing fever and eventually anaemia and reduced amounts of blood flow to critical organs.
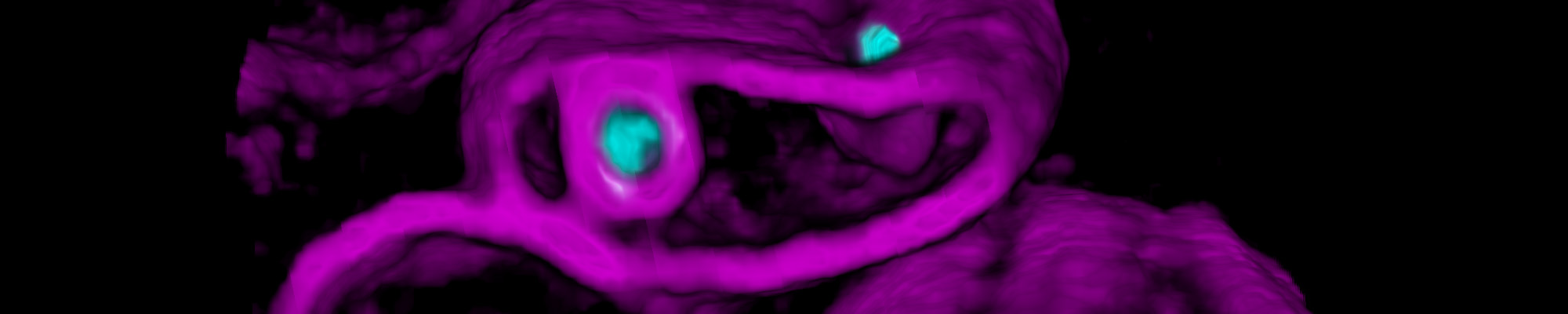
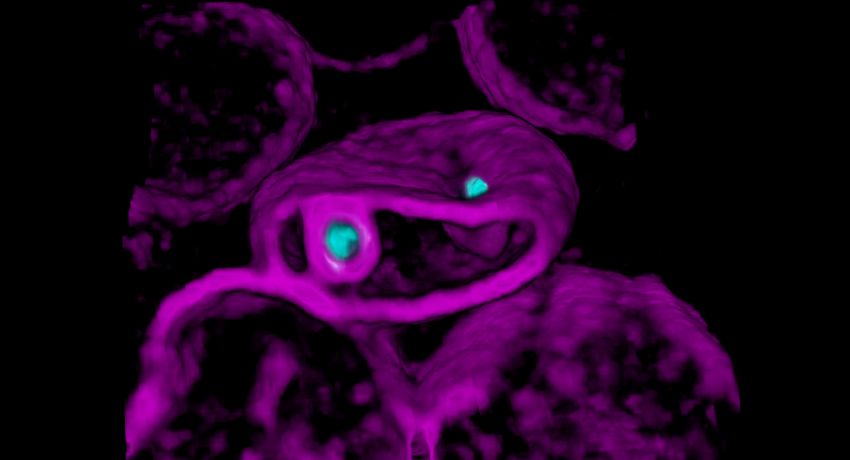
Researchers have been working on understanding how the malaria parasite invades red blood cells as a strategy for designing vaccines or drugs that block this important stage of the parasite’s life cycle. Advanced live-microscopy techniques have become the most effective avenue for studying the interactions between the malaria parasite and the host red blood cell prior to, during, and after infection.
A red blood cell (magenta) is being invaded by two malaria parasites (cyan), where each parasite wraps itself with the red blood cell membrane to form a ‘pocket’ in which it will grow and replicate. Credit: Ms Cindy Evelyn and Dr Niall Geoghegan, Rogers Laboratory
How malaria parasites break into red blood cells
Unlike many other cells, the red blood cell is not endocytic, meaning that it does not have a natural way for bringing in material from the outside environment.

In order for the malaria parasite to enter its host red blood cell, significant changes to the red blood cell membrane must take place. Understanding this complex interplay between the parasite and red blood cell is the key to determining drug targets that block the process effectively.
Live-imaging is the most non-destructive way to study such biological events, but the light-sensitive, rapid, and stochastic nature of the invasion process pose a challenge to this approach.
Furthermore, the malaria parasite is quite small, only one micron in size when it transforms into the blood stage invasive state.
Lattice light-sheet microscopy sheds light on the invasion process
Cindy Evelyn, a research assistant in the laboratory of Associate Professor Kelly Rogers, established a fluorescence live-imaging protocol that enables her to record malaria parasite invasion of red blood cells in three dimensions.
Under the supervision of Dr Niall Geoghegan and Associate Professor Rogers, Ms Evelyn uses lattice light sheet microscopy to acquire high-resolution timelapse images of invasion events. This technique allows gentle illumination and produces data with sufficient resolution for performing quantitative analyses on the host-pathogen interactions.
With the help of Dr Lachlan Whitehead and Dr Pradeep Rajasekhar, Ms Evelyn also establishes sophisticated data analysis pipelines that include automation and machine learning to extract useful information from the imaging data. In collaboration with Professor Alan Cowman’s laboratory, the team are using this method to fill in the gaps on the multi-step process of blood stage malaria parasite invasion.
When a closer look is necessary
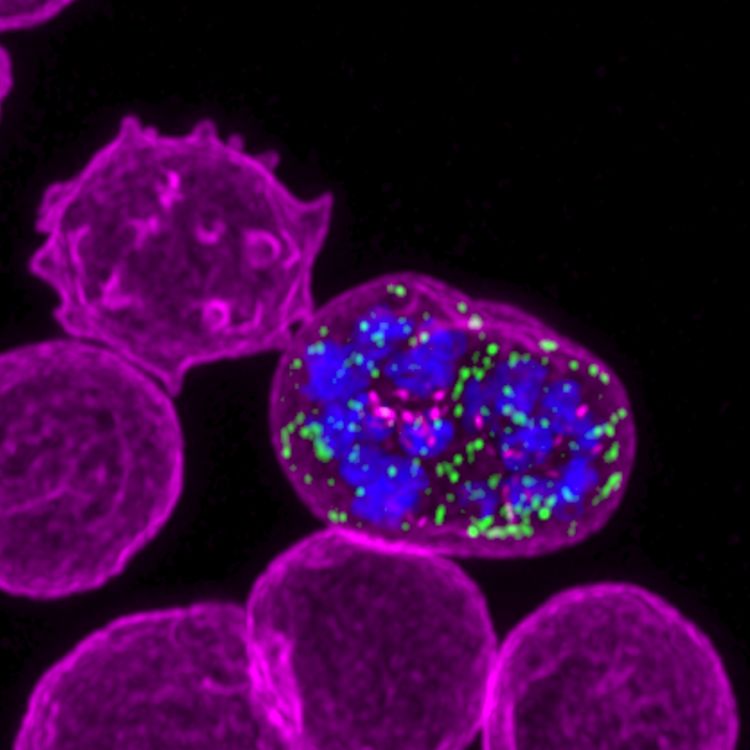
At times, higher resolution is needed to observe the localisation of specific proteins or molecules during the invasion process. Fortunately, the Centre for Dynamic Imaging offers a wide range of microscopy platforms that cover many different needs. With help from Dr Michael Mlodzianoski in performing three-dimensional structured illumination microscopy (3D-SIM) on the DeltaVision OMX SR, Ms Evelyn visualises the localisation of RON3 protein at the parasite’s rhoptry, a secretory organelle that plays a substantial role in the invasion process.
This super-resolution technique complements the live-imaging approach in piecing together the concerted molecular and biophysical events in the malaria invasion process.
“The lattice light sheet microscope allows me to observe the order of events in this intricate invasion process, while super-resolution microscopy gives me access to a more detailed snapshot of important timepoints throughout the process,” says Ms Evelyn.
Studying invasion phenotype
Having painted a clear picture of the invasion process through visualisation and quantitative parameters, the team are now equipped to define and interrogate any abnormality in the invasion process for drug-treated conditions or genetically modified parasite lines.
“We now have a way of understanding which protein-protein interaction plays which role in invasion and how an inhibitor blocks the invasion process," Ms Evelyn says. "To target the enemy effectively is to know its strategies!”