Blood vessel growth is essential for normal development but can also contribute to cancer and a spectrum of vision-impairing diseases.
Researchers are using advanced imaging techniques to investigate the molecular regulation of blood vessel growth, with a particular focus on the role of programmed cell death.
Improving our understanding of how blood vessels grow may open up new avenues for therapy.
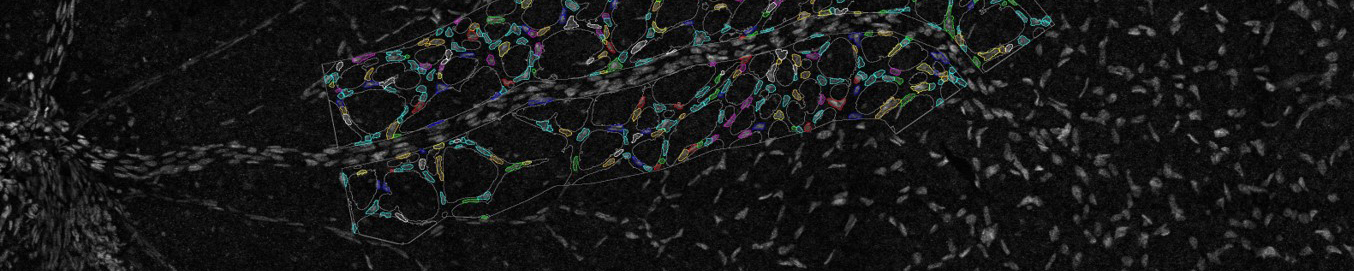
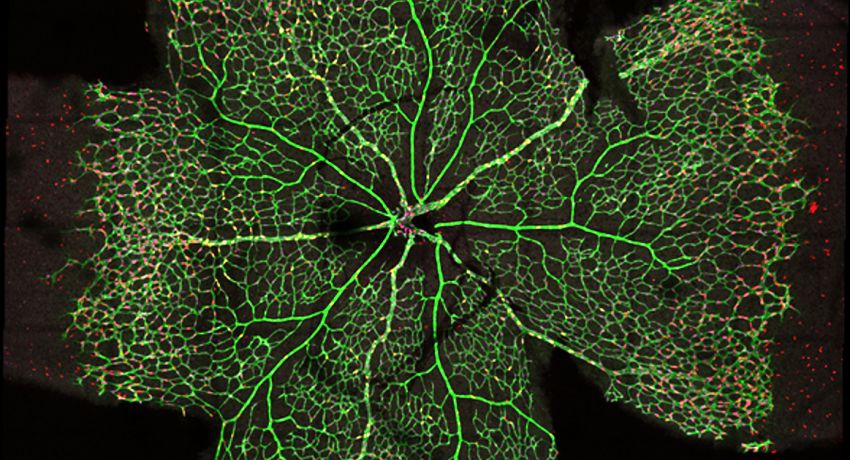
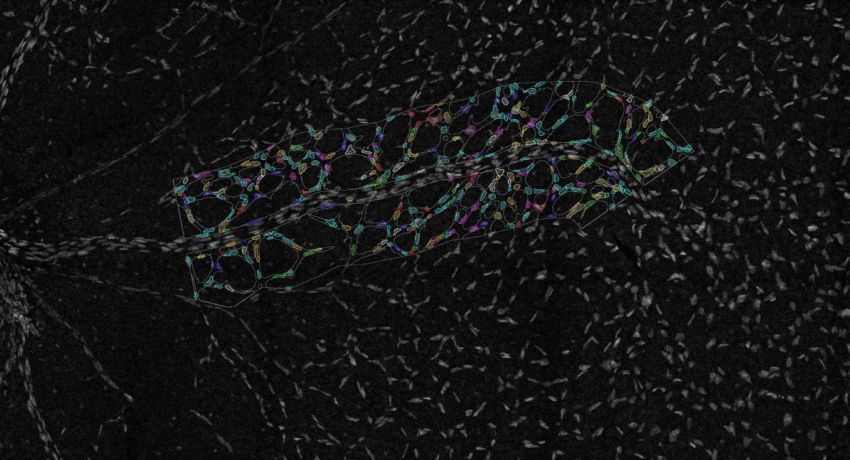
Imaging of the retinal vasculature is used to study various eye diseases and conditions. Here cells within the minor vasculature are segmented while the main vessels are excluded. Credit: Emma Watson, Leigh Coultas, Lachlan Whitehead
Angiogenesis is poorly understood

Blood vessels develop through a process termed ‘angiogenesis’: the growth of new vessels from existing vessels. While angiogenesis is vital for normal development, inappropriate vessel growth can contribute to cancer and eye diseases such as age-related macular degeneration and diabetic retinopathy – two leading causes of blindness in adults.
Despite the critical role of angiogenesis in development and disease, little is known about how this process is regulated.
Dr Coultas is particularly interested in the role of programmed cell death, or apoptosis, in angiogenesis.
During angiogenesis, new vessel growth is accompanied by the selective pruning of redundant vessel segments to generate a tree-like network capable of efficient blood distribution. For a long time, scientists assumed that apoptosis controls vessel pruning but no techniques were available to formally investigate this idea.
A workhorse for studying angiogenesis
Advanced imaging techniques are allowing Dr Coultas to directly observe the role of apoptosis in angiogenesis. Using the Leica SP8 Resonant Scanning Confocal microscope, Dr Coultas obtains three-dimensional data of the entire retina, revealing how the blood vessels develop into a complex tree-like structure.
He performs quantitative experiments, measuring parameters including:
- the number of dying cells in the vessels
- the density of the blood vessels
- the size, shape, length and branch points of the vessels
- whether the vessels are growing or regressing
This information can be used to compare different disease models and investigate the effect of blocking or promoting apoptosis. The imaging team provides expertise with data analysis and provides help when Dr Coultas is establishing a new imaging protocol, such as setting up the parameters for live imaging experiments.
Apoptosis is dispensable for vessel pruning
Surprisingly, Dr Coultas found that blocking apoptosis did not prevent vessel pruning during angiogenesis. By carefully examining blood vessel characteristics in different parts of the retina, he found that apoptosis helps remove vessel segments that have already been pruned off, and influences the diameter of specific types of blood vessels.
Ultimately, Dr Coultas believes that understanding how angiogenesis is regulated will lead to new ways of manipulating vascular growth or regression for therapeutic benefit.